Background: JAK2 inhibitor ruxolitinib leads to improvements in symptom burden and spleen volume in patients with myelofibrosis (MF), but suboptimal response represents a major unmet clinical need. Deregulated inflammatory pathways including CXCR4/CXCR12 axis might limit the therapeutic efficacy of ruxolitinib and contribute to disease progression ( Miwa et al. Pathology. 2013), where agents targeting bone marrow (BM) inflammation could be promising. Adoptive therapy with allogeneic, cord blood-derived regulatory T cells (Tregs) (CK0801- HLA 3/ 6 match, fresh infusion) showed safety and early clinical signal in MF ( Kadia et al., Blood. 2020). In preclinical studies, the next generation product, CK0804, consisting of CXCR4 enriched Tregs [CK0804] shows preferential homing to BM and decrease inflammatory cytokines including TGFα, TNFβ, IL-13 and TGFβ when compared to unmanipulated control Treg cells ( Huang et al., Cytotherapy, 2023).
Methods:This phase Ib study evaluates the safety and activity of CK0804 (non-HLA matched, Cryopreserved, Multi-Dose) Treg therapy in patients with suboptimal response to ruxolitinib. The study design consists of a safety run-in phase of 9 patients followed by an expansion of additional 15 patients. Participants will continue ruxolitinib and receive infusion of CK0804 at a fixed dose of 100 million Treg cells every 28 days up to 6 cycles. All patients are monitored for 6 months following the last infusion. Patients with MF on ruxolitinib for ≥12 weeks and stable dose for ≥8 weeks, who have palpable splenomegaly, symptoms or grade 2 cytopenia are eligible. Primary objective is safety, secondary objective includes overall response per IWG-MRT criteria at 24 weeks. Exploratory objectives evaluate pharmacodynamics, pharmacokinetics, markers of immunogenicity and inflammation.
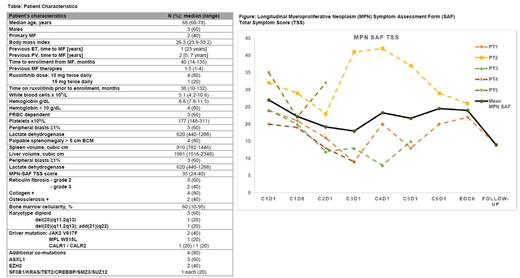
Results: As of 1 st of July 2023, 5 patients were enrolled and treated with a median age of 68 years [range 60-78], 60% males (table). Median number of previous MF therapies was 1.5 (range 1-4), median duration of previous ruxolitinib therapy was 38 months (range, 10-132). At enrollment, all patients were symptomatic with median MPN-SAF TSS of 35 (range, 24-40), 3 patients had worsening splenomegaly and 3 patients were transfusion dependent. All patients had grade 2+ reticulin marrow fibrosis with most also having significant collagen or osteosclerosis, 40% were JAK2 V617F mutated and 80% had additional co-mutations (table).
Two (2) patients received all six (6) doses of CK0804, two patients have ongoing treatments: one patient received 5 doses and one patient received 3 doses. One patient has withdrawn consent after receiving 2 doses. This patient experienced an infusion reaction to her second dose of CK0804 likely due to the excipient dimethylsulfoxide (DMSO) used for cryopreservation of cells. All other patients tolerated their infusions well with no adverse reactions. No hematologic adverse events were observed. Ruxolitinib dose remained unchanged in all patients throughout the study.
Among the three (3) patients who were transfusion dependent, two (2) were evaluable for response. Their monthly transfusion requirements by the end of the sixth (6 th) cycle improved from the baseline value of four (4) units and 1.2 units to 2.8 and 0.8 units, respectively. Spleen volume at four (4) months assessment (3 patients) remained stable. MPN-SAF TSS showed improvement in all patients on therapy, although it requires longer follow-up (figure). Correlative studies including longitudinal analysis of inflammatory cytokines and immune reconstitution will be presented at the conference.
Conclusion: The preliminary analysis of this study evaluating CK0804 (CXCR4 enriched T regs cell therapy) as addition to ruxolitinib shows initial safety with no myelosuppressive adverse events and promising clinical activity. The study is actively enrolling participants (NCT05423691).
MD Anderson Cancer Center has an institutional conflict of interest with Cellenkos related to the research presented herein. Institutional Conflict of Interest Management and Monitoring Plan is being implemented with respect to MD Anderson Cancer Center's conduct of this research.
Disclosures
Masarova:MorphoSys US: Membership on an entity's Board of Directors or advisory committees. Pemmaraju:Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karger Publishers: Other: Licenses; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; United States Department of Defense (DOD): Research Funding; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASCO Cancer.Net Editorial Board: Other: Leadership; ASH Committee on Communications: Other: Leadership; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kadia:Pulmotect, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Iterion: Research Funding; Novartis: Consultancy; Regeneron Pharmaceuticals: Research Funding; Cellenkos Inc.: Research Funding; Janssen Research and Development: Research Funding; Cure: Speakers Bureau; SELLAS Life Sciences Group: Research Funding; Liberum: Consultancy; GenFleet Therapeutics: Research Funding; Amgen, Inc.: Research Funding; Sanofi-Aventis: Consultancy; Delta-Fly Pharma, Inc.: Research Funding; Celgene: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Cyclacel: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Ascentage Pharma Group: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Genzyme: Honoraria; AstraZeneca: Research Funding; Genentech: Consultancy, Research Funding; Astellas Pharma Global Development: Research Funding; Glycomimetics: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Bose:Incyte, BMS, CTI, Morphosys, Blueprint, Cogent, Sumitomo: Honoraria, Research Funding; GSK, Novartis, Karyopharm, AbbVie, Pharma Essentia, Jubilant, Morphic: Honoraria; Kartos, Telios, Ionis, Disc, Janssen, Geron: Research Funding. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. Sadeghi:Cellenkos Inc: Current Employment, Current equity holder in private company. Asatiani:Incyte: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Parmar:Cellenkos Inc: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Flowers:N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Karyopharm: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics: Research Funding; CPRIT Scholar in Cancer Research: Research Funding; Pharmacyclics Jansen: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Pfizer: Research Funding; Genmab: Consultancy; Genentech Roche: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Beigene: Consultancy; Spectrum: Consultancy; Denovo Biopharma: Consultancy; Ziopharm: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; Gilead: Consultancy, Research Funding; V Foundation: Research Funding; National Cancer Institute: Research Funding; SeaGen: Consultancy; Guardant: Research Funding; Kite: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Novartis: Research Funding; Nektar: Research Funding; Burroghs Wellcome Fund: Research Funding; Acerta: Research Funding; 4D: Research Funding; Morphosys: Research Funding; Jannsen Pharmaceuticals: Research Funding; Iovance: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal